|
|
 |
|
May 2005 Scientific
American
|
His Brain, Her Brain |
|
It turns out that male and female brains differ quite a bit in architecture and activity. Research into these variations
could lead to sex-specific treatments for disorders such as depression and schizophrenia |
|
By Larry Cahill |
|
 |
|
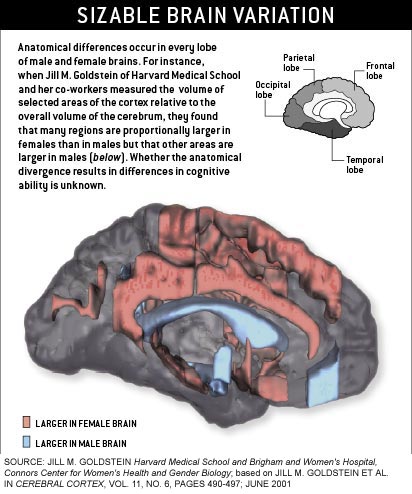
Scientists sizing up the brains of both sexes began using their main finding--that female brains tend to be smaller--to
bolster the view that women are intellectually inferior to men.
To date, no
one has uncovered any evidence that anatomical disparities might render women incapable of achieving academic distinction in math, physics or engineering. And the brains of men and women have been
shown to be quite clearly similar in many ways. Nevertheless, over the past decade investigators have documented an astonishing
array of structural, chemical and functional variations in the brains of males and females.
|
These inequities are not just interesting idiosyncrasies that might explain why more men than women enjoy the Three
Stooges. They raise the possibility that we might need to develop sex-specific treatments for a host of conditions, including
depression, addiction, schizophrenia and post-traumatic stress disorder (PTSD). Furthermore, the differences imply that researchers
exploring the structure and function of the brain must take into account the sex of their subjects when analyzing their data--and
include both women and men in future studies or risk obtaining misleading results.
Sculpting the Brain
Not so long ago neuroscientists believed that sex differences
in the brain were limited mainly to those regions responsible for mating behavior. In a 1966 Scientific American article
entitled "Sex Differences in the Brain," Seymour Levine of Stanford University described how sex hormones help to direct divergent
reproductive behaviors in rats--with males engaging in mounting and females arching their backs and raising their rumps to
attract suitors. Levine mentioned only one brain region in his review: the hypothalamus, a small structure at the base of
the brain that is involved in regulating hormone production and controlling basic behaviors such as eating, drinking and sex.
A generation of neuroscientists came to maturity believing that "sex differences in the brain" referred primarily to mating
behaviors, sex hormones and the hypothalamus.
Differences in the size of brain
structures are generally thought to reflect their relative importance to the animal. For example, primates rely more on vision
than olfaction; for rats, the opposite is true. As a result, primate brains maintain proportionately larger regions devoted
to vision, and rats devote more space to olfaction. So the existence of widespread anatomical disparities between men and
women suggests that sex does influence the way the brain works.
Other investigations are finding
anatomical sex differences at the cellular level. For example, Sandra Witelson and her colleagues at McMaster University discovered
that women possess a greater density of neurons in parts of the temporal lobe cortex associated with language processing and
comprehension. On counting the neurons in postmortem samples, the researchers found that of the six layers present in the
cortex, two show more neurons per unit volume in females than in males. Similar findings were subsequently reported for the
frontal lobe. With such information in hand, neuroscientists can now explore whether sex differences in neuron number correlate
with differences in cognitive abilities--examining, for example, whether the boost in density in the female auditory cortex
relates to women's enhanced performance on tests of verbal fluency.
Differences in the size of brain
structures are generally thought to reflect their relative importance to the animal. For example, primates rely more on vision
than olfaction; for rats, the opposite is true. As a result, primate brains maintain proportionately larger regions devoted
to vision, and rats devote more space to olfaction. So the existence of widespread anatomical disparities between men and
women suggests that sex does influence the way the brain works.
Other investigations are finding
anatomical sex differences at the cellular level. For example, Sandra Witelson and her colleagues at McMaster University discovered
that women possess a greater density of neurons in parts of the temporal lobe cortex associated with language processing and
comprehension. On counting the neurons in postmortem samples, the researchers found that of the six layers present in the
cortex, two show more neurons per unit volume in females than in males. Similar findings were subsequently reported for the
frontal lobe. With such information in hand, neuroscientists can now explore whether sex differences in neuron number correlate
with differences in cognitive abilities--examining, for example, whether the boost in density in the female auditory cortex
relates to women's enhanced performance on tests of verbal fluency.
Such anatomical diversity may be caused in large part by the activity of the sex hormones that bathe the fetal brain.
These steroids help to direct the organization and wiring of the brain during development and influence the structure and
neuronal density of various regions. Interestingly, the brain areas that Goldstein found to differ between men and women are
ones that in animals contain the highest number of sex hormone receptors during development. This correlation between brain
region size in adults and sex steroid action in utero suggests that at least some sex differences in cognitive function do
not result from cultural influences or the hormonal changes associated with puberty--they are there from birth.
Inborn Inclinations
Several intriguing behavioral studies add to the evidence
that some sex differences in the brain arise before a baby draws its first breath. Through the years, many researchers have
demonstrated that when selecting toys, young boys and girls part ways. Boys tend to gravitate toward balls or toy cars, whereas
girls more typically reach for a doll. But no one could really say whether those preferences are dictated by culture or by
innate brain biology.
#1
To address this question, Melissa Hines of City University London and Gerianne M. Alexander of Texas A&M University
turned to monkeys, one of our closest animal cousins. The researchers presented a group of vervet monkeys with a selection
of toys, including rag dolls, trucks and some gender-neutral items such as picture books. They found that male monkeys spent
more time playing with the "masculine" toys than their female counterparts did, and female monkeys spent more time interacting
with the playthings typically preferred by girls. Both sexes spent equal time monkeying with the picture books and other gender-neutral
toys.
Because vervet monkeys are unlikely
to be swayed by the social pressures of human culture, the results imply that toy preferences in children result at least
in part from innate biological differences. This divergence, and indeed all the anatomical sex differences in the brain, presumably
arose as a result of selective pressures during evolution. In the case of the toy study, males--both human and primate--prefer
toys that can be propelled through space and that promote rough-and-tumble play. These qualities, it seems reasonable to speculate,
might relate to the behaviors useful for hunting and for securing a mate. Similarly, one might also hypothesize that females,
on the other hand, select toys that allow them to hone the skills they will one day need to nurture their young.
Simon Baron-Cohen and his associates
at the University of Cambridge took a different but equally creative approach to addressing the influence of nature versus
nurture regarding sex differences. Many researchers have described disparities in how "people-centered" male and female infants
are. For example, Baron-Cohen and his student Svetlana Lutchmaya found that one-year-old girls spend more time looking at
their mothers than boys of the same age do. And when these babies are presented with a choice of films to watch, the girls
look longer at a film of a face, whereas boys lean toward a film featuring cars.
|
Of course, these preferences
might be attributable to differences in the way adults handle or play with boys and girls. To eliminate this possibility,
Baron-Cohen and his students went a step further. They took their video camera to a maternity ward to examine the preferences
of babies that were only one day old. The infants saw either the friendly face of a live female student or a mobile that matched
the color, size and shape of the student's face and included a scrambled mix of her facial features. To avoid any bias, the
experimenters were unaware of each baby's sex during testing. When they watched the tapes, they found that the girls spent
more time looking at the student, whereas the boys spent more time looking at the mechanical object. This difference in social
interest was evident on day one of life--implying again that we come out of the womb with some cognitive sex differences built
in.
Under Stress
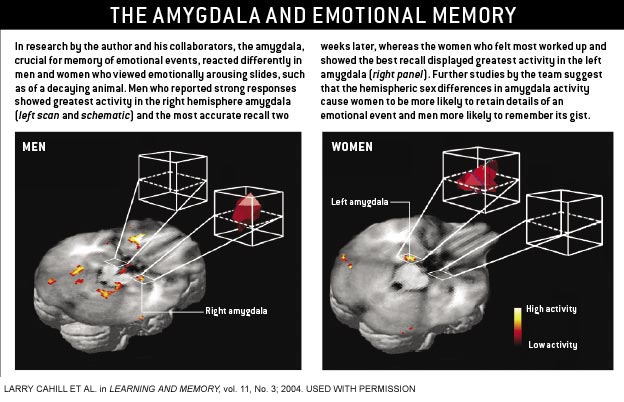
In many cases, sex differences in the brain's chemistry and
construction influence how males and females respond to the environment or react to, and remember, stressful events. Take,
for example, the amygdala. Goldstein and others have reported that the amygdala is larger in men than in women. And in rats,
the neurons in this region make more numerous interconnections in males than in females. These anatomical variations would
be expected to produce differences in the way that males and females react to stress. |
|
To assess whether male and female amygdalae in fact respond differently to stress, Katharina Braun and her co-workers
at Otto von Guericke University in Magdeburg, Germany, briefly removed a litter of Degu pups from their mother. For these
social South American rodents, which live in large colonies like prairie dogs do, even temporary separation can be quite upsetting.
The researchers then measured the concentration of serotonin receptors in various brain regions. Serotonin is a neurotransmitter,
or signal-carrying molecule, that is key for mediating emotional behavior. (Prozac, for example, acts by increasing serotonin
function.)
|
The workers allowed the pups
to hear their mother's call during the period of separation and found that this auditory input increased the serotonin receptor
concentration in the males' amygdala, yet decreased the concentration of these same receptors in females. Although it is difficult
to extrapolate from this study to human behavior, the results hint that if something similar occurs in children, separation
anxiety might differentially affect the emotional well-being of male and female infants. Experiments such as these are necessary
if we are to understand why, for instance, anxiety disorders are far more prevalent in girls than in boys. |
|
Another brain region now known to diverge in the sexes anatomically and in its response to stress is the hippocampus,
a structure crucial for memory storage and for spatial mapping of the physical environment. Imaging consistently demonstrates
that the hippocampus is larger in women than in men. These anatomical differences might well relate somehow to differences
in the way males and females navigate. Many studies suggest that men are more likely to navigate by estimating distance in
space and orientation ("dead reckoning"), whereas women are more likely to navigate by monitoring landmarks. Interestingly,
a similar sex difference exists in rats. Male rats are more likely to navigate mazes using directional and positional information,
whereas female rats are more likely to navigate the same mazes using available landmarks. (Investigators have yet to demonstrate,
however, that male rats are less likely to ask for directions.)
Even the neurons in the hippocampus
behave differently in males and females, at least in how they react to learning experiences. For example, Janice M. Juraska
and her associates at the University of Illinois have shown that placing rats in an "enriched environment"--cages filled with
toys and with fellow rodents to promote social interactions--produced dissimilar effects on the structure of hippocampal neurons
in male and female rats. In females, the experience enhanced the "bushiness" of the branches in the cells' dendritic trees--the
many-armed structures that receive signals from other nerve cells. This change presumably reflects an increase in neuronal
connections, which in turn is thought to be involved with the laying down of memories. In males, however, the complex environment
either had no effect on the dendritic trees or pruned them slightly. |
|
|
But male rats sometimes learn better in the face of stress. Tracey J. Shors of Rutgers University and her collaborators
have found that a brief exposure to a series of one-second tail shocks enhanced performance of a learned task and increased
the density of dendritic connections to other neurons in male rats yet impaired performance and decreased connection density
in female rats. Findings such as these have interesting social implications. The more we discover about how brain mechanisms
of learning differ between the sexes, the more we may need to consider how optimal learning environments potentially differ
for boys and girls.
|
 |
|
|
 |
|
|
 |
|
|
|
|
|
Although the hippocampus of
the female rat can show a decrement in response to acute stress, it appears to be more resilient than its male counterpart
in the face of chronic stress. Cheryl D. Conrad and her co-workers at Arizona State University restrained rats in a mesh cage
for six hours--a situation that the rodents find disturbing. The researchers then assessed how vulnerable their hippocampal
neurons were to killing by a neurotoxin--a standard measure of the effect of stress on these cells. They noted that chronic
restraint rendered the males' hippocampal cells more susceptible to the toxin but had no effect on the females' vulnerability.
These findings, and others like them, suggest that in terms of brain damage, females may be better equipped to tolerate chronic
stress than males are. Still unclear is what protects female hippocampal cells from the damaging effects of chronic stress,
but sex hormones very likely play a role. |
|
The Big Picture
Extending the work on how the brain handles and remembers
stressful events, my colleagues and I have found contrasts in the way men and women lay down memories of emotionally arousing
incidents--a process known from animal research to involve activation of the amygdala. In one of our first experiments with
human subjects, we showed volunteers a series of graphically violent films while we measured their brain activity using PET.
A few weeks later we gave them a quiz to see what they remembered.
We discovered that the number
of disturbing films they could recall correlated with how active their amygdala had been during the viewing. Subsequent work
from our laboratory and others confirmed this general finding. But then I noticed something strange. The amygdala activation
in some studies involved only the right hemisphere, and in others it involved only the left hemisphere. It was then I realized
that the experiments in which the right amygdala lit up involved only men; those in which the left amygdala was fired up involved
women. Since then, three subsequent studies--two from our group and one from John Gabrieli and Turhan Canli and their collaborators
at Stanford--have confirmed this difference in how the brains of men and women handle emotional memories.
|
The realization that male and female brains were processing the same emotionally arousing material into memory differently
led us to wonder what this disparity might mean. To address this question, we turned to a century-old theory stating that
the right hemisphere is biased toward processing the central aspects of a situation, whereas the left hemisphere tends to
process the finer details. If that conception is true, we reasoned, a drug that dampens the activity of the amygdala should
impair a man's ability to recall the gist of an emotional story (by hampering the right amygdala) but should hinder a woman's
ability to come up with the precise details (by hampering the left amygdala).
Propranolol is such a drug.
This so-called beta blocker quiets the activity of adrenaline and its cousin noradrenaline and, in so doing, dampens the activation
of the amygdala and weakens recall of emotionally arousing memories. We gave this drug to men and women before they viewed
a short slide show about a young boy caught in a terrible accident while walking with his mother. One week later we tested
their memory. The results showed that propranolol made it harder for men to remember the more holistic aspects, or gist, of
the story--that the boy had been run over by a car, for example. In women, propranolol did the converse, impairing their memory
for peripheral details--that the boy had been carrying a soccer ball. |
In more recent investigations,
we found that we can detect a hemispheric difference between the sexes in response to emotional material almost immediately.
Volunteers shown emotionally unpleasant photographs react within 300 milliseconds--a response that shows up as a spike on
a recording of the brain's electrical activity. With Antonella Gasbarri and others at the University of L'Aquila in Italy,
we have found that in men, this quick spike, termed a P300 response, is more exaggerated when recorded over the right hemisphere;
in women, it is larger when recorded over the left. Hence, sex-related hemispheric disparities in how the brain processes
emotional images begin within 300 milliseconds--long before people have had much, if any, chance to consciously interpret
what they have seen.
|
These discoveries might have
ramifications for the treatment of PTSD. Previous research by Gustav Schelling and his associates at Ludwig Maximilian University
in Germany had established that drugs such as propranolol diminish memory for traumatic situations when administered as part
of the usual therapies in an intensive care unit. Prompted by our findings, they found that, at least in such units, beta
blockers reduce memory for traumatic events in women but not in men. Even in intensive care, then, physicians may need to
consider the sex of their patients when meting out their medications.
|
Sex and Mental Disorders
ptsd is not the only psychological
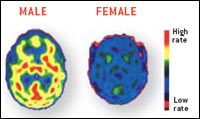
disturbance that appears to play out differently in women and men. A PET study by Mirko Diksic and his colleagues at McGill
University showed that serotonin production was a remarkable 52 percent higher on average in men than in women, which might
help clarify why women are more prone to depression--a disorder commonly treated with drugs that boost the concentration of
serotonin.
A similar situation might prevail
in addiction. In this case, the neurotransmitter in question is dopamine--a chemical involved in the feelings of pleasure
associated with drugs of abuse. Studying rats, Jill B. Becker and her fellow investigators at the University of Michigan at
Ann Arbor discovered that in females, estrogen boosted the release of dopamine in brain regions important for regulating drug-seeking
behavior. Furthermore, the hormone had long-lasting effects, making the female rats more likely to pursue cocaine weeks after
last receiving the drug. Such differences in susceptibility--particularly to stimulants such as cocaine and amphetamine--could
explain why women might be more vulnerable to the effects of these drugs and why they tend to progress more rapidly from initial
use to dependence than men do.
|
|
Certain brain abnormalities underlying schizophrenia appear to differ in men and women as well. Ruben Gur, Raquel Gur
and their colleagues at the University of Pennsylvania have spent years investigating sex-related differences in brain anatomy
and function. In one project, they measured the size of the orbitofrontal cortex, a region involved in regulating emotions,
and compared it with the size of the amygdala, implicated more in producing emotional reactions. The investigators found that
women possess a significantly larger orbitofrontal-to-amygdala ratio (OAR) than men do. One can speculate from these findings
that women might on average prove more capable of controlling their emotional reactions.
In additional experiments, the
researchers discovered that this balance appears to be altered in schizophrenia, though not identically for men and women.
Women with schizophrenia have a decreased OAR relative to their healthy peers, as might be expected. But men, oddly, have
an increased OAR relative to healthy men. These findings remain puzzling, but, at the least, they imply that schizophrenia
is a somewhat different disease in men and women and that treatment of the disorder might need to be tailored to the sex of
the patient.
Sex Matters
in a comprehensive 2001 report on sex differences in human
health, the prestigious National Academy of Sciences asserted that "sex matters. Sex, that is, being male or female, is an
important basic human variable that should be considered when designing and analyzing studies in all areas and at all levels
of biomedical and health-related research." |
Neuroscientists are still far
from putting all the pieces together--identifying all the sex-related variations in the brain and pinpointing their influences
on cognition and propensity for brain-related disorders. Nevertheless, the research conducted to date certainly demonstrates
that differences extend far beyond the hypothalamus and mating behavior. Researchers and clinicians are not always clear on
the best way to go forward in deciphering the full influences of sex on the brain, behavior and responses to medications.
But growing numbers now agree that going back to assuming we can evaluate one sex and learn equally about both is no longer
an option.
|
LARRY CAHILL received his Ph.D. in neuroscience in 1990 from the University of California, Irvine. After spending two
years in Germany using imaging techniques to explore learning and memory in gerbils, he returned to U.C. Irvine, where he
is now an associate professor in the department of neurobiology and behavior and a Fellow of the Center for the Neurobiology
of Learning and Memory. |
|
|
|
|
|
|
|
|
|
 |

