|
An FDA study on rupture of silicone gel-filled breast implants was presented at the Sixth World
Biomaterials Congress on May 18, 2000.
The study, performed in Birmingham, Alabama, involved women who had their first breast implant before 1988.
The majority of the 907 women in this study had silicone gel-filled breast implants. Some women who had silicone gel-filled
breast implants were invited to undergo a magnetic resonance imaging (MRI) examination of their breasts to determine whether
their implants had ruptured. MRI allows the radiologist to see the breast implant while it is still inside the breast.
FDA conducted this study because of concerns about the frequency and results of rupture. Rupture is a concern
because:
- Rupture of silicone gel-filled implants may allow silicone to migrate through the tissues. The
relationship of free silicone to development or progression of disease is unknown.
- Rupture is a device failure – the implant is no longer performing as intended.
Protocol
- Women in this study were identified because they had been in a National Cancer Institute study
on women with breast implants. Women who responded to a questionnaire in the NCI study were eligible for this study. This
cohort included only women who were patients at two plastic surgery practices. The 907 women in this study were a subset of
1247 women in the Birmingham, Ala., area who were part of an NCI study on breast implants.
- The rupture study had two parts. In the first part, 907 women were interviewed about surgeries
in which implant(s) were surgically removed (interview component of the study).
- One third of the 907 women in the first part of the study reported that they
had at least one surgery in which their implant was removed and replaced. Women were also asked the main reason they had their
implants removed, and if an implant rupture was suspected prior to the surgery.
- If women reported that their implant surgery was for a suspected implant rupture, they were asked
about symptoms that they may have had and about whether they knew of a possible cause of the rupture.
- In the second part of the study, 344 women with silicone gel breast implants received MRI examinations
to detect possible implant rupture. The women, selected randomly from the first part of the study, were invited to have MRI exams when they were called to be in the study until all the MRI
appointments planned for the study had been filled. The study had funding for up to 400 MRI exams to be accomplished
at a particular MRI facility under contract for a certain period of time. Of the 445 women invited to have an MRI, 80% (359)
accepted and had the examination. Women were invited for the MRI exam without regard to whether they had symptoms of breast
implant rupture. Those who accepted the appointments and came were no more likely to think their breast implant was ruptured
than women who declined the examination or did not come to their appointments.
- The 344 women who received MRI examinations had a total of 687 implants. The
average age of the women in the MRI cohort was 51 ± 8 years. Most women in this cohort had a single lumen gel breast implant (82%) and the remainder had
a double-lumen gel breast implant (silicone and saline). For the 677 implants for which this information was available, the
average implant age was 17 ± 3 years.
- Three independent radiologists reviewed the results of all of the women’s MRIs and for each
implant, determined whether it was intact, indeterminate (suspicious for rupture), or ruptured. The agreement between the
radiologists was very high.
Results
- At least two of the three study radiologists agreed that 378 of the 687
implants were ruptured (55%). This means that 69% of the 344 women had at least one ruptured breast implant.
- Radiologists observed that silicone gel had leaked outside the fibrous scar capsule that forms
around the implant in 85 of the 687 implants (12%). Of the 344 women, 73 (21%) had silicone gel outside the capsule
in one or both breasts.
- Factors that were associated with rupture: the age of the implant, the implant manufacturer, and
whether the device was implanted above or beneath the chest muscles.
Limitations of the Study
- This cohort included only women who were at two plastic surgery practices. It is unknown whether
the results of the study might have been different if patients from other parts of the U.S. had been included.
- Only 80% of those invited to have an MRI examination agreed.
- Many types of silicone gel-filled breast implants were included in this study.
- While MR imaging is considered the best method for imaging breast implants for rupture, it is not
perfect.
Conclusion
- MRI examination in this cohort of patients demonstrated that the majority of women had at least
one ruptured implant.
Funding and Authors
- Funding for this study came from: the Office of Women’s Health, FDA; the Office of the Commissioner;
the National Cancer Institute, NIH; the Office of Research on Women’s Health, NIH; and the U.S. Department of Health
and Human Services.
- Authors were S. Lori Brown, PhD, MPH , Michael S. Middleton, PhD, MD 2 , Wendie A. Berg,
MD, PhD 3 , Mary Scott Soo, MD 4, and Gene Pennello, PhD 1.
1Office of Surveillance and Biometrics, Center for Devices and Radiological Health, Food and Drug Administration,
Rockville, MD 20850
2Department
of Radiology, University of California at San Diego School of Medicine, San Diego, CA 92103
3Division
of Breast Imaging, Department of Radiology and Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore,
MD 21201
4Department
of Radiology, Duke South Hospital, Duke University Medical Center, Durham, NC 27710
Back to Breast Implant Information Page
From FDA Breast Implant Consumer Handbook 2004 at http://www.fda.gov/cdrh/breastimplants/handbook2004
/localcomplications.html#3
LOCAL COMPLICATIONS & REOPERATIONS
The Institute of Medicine (IOM) completed its independent review of past and ongoing scientific
research of silicone [both saline-filled and silicone-gel filled] breast implant safety in June 1999.6 Below are
some of the major findings from the IOM report.
- Local complications are the primary safety issue with breast implants because they are frequent enough
to be a concern.
- Local complications accumulate over the lifetime of the implant, and they have not been well studied.
(ABOVE STUDY SHOWS A 55% RISK OF RUPTURE—jk)
- Information on local complications is crucial for women deciding whether or not they want breast
implants.
Key points to consider whether you are undergoing breast augmentation, reconstruction, or revision:
- Breast implants will not last a lifetime. Either because of rupture or other complications,
you will likely need to have the implants removed.
- You are likely to need additional doctor visits and reoperations because of one or more complications
over the course of your life.
- You are likely to have the implants removed, with or without replacement, because of one or more
complications over the course of your life.
- Many of the changes to your breast following implantation may be cosmetically undesirable, as well
as irreversible (cannot be undone).
- If you later choose to have your implants removed, you may experience unacceptable dimpling, puckering,
wrinkling, loss of breast tissue, or other undesirable cosmetic changes of the breast.
Potential local breast implant complications are bulleted below. You may need non-surgical treatments or reoperations
(including removal of your implant) to treat any of these local complications. Potential local complications include, but
are not limited to:
|
• Asymmetry |
• Inflammation/irritation |
|
• Breast pain |
• Malposition/displacement |
|
• Breast tissue atrophy |
• Necrosis |
|
• Calcification/calcium deposits |
• Nipple/breast sensation changes |
|
• Capsular contracture |
• Palpability/visibility |
|
• Chest wall deformity |
• Ptosis |
|
• Delayed wound healing |
• Redness/bruising |
|
• Extrusion |
• Rupture/deflation |
|
• Galactorrhea |
• Scarring |
|
• Granuloma |
• Seroma |
|
• Hematoma |
• Unsatisfactory style/size |
|
• Iatrogenic injury/damage |
• Wrinkling/rippling |
|
• Infection, including Toxic Shock Syndrome |
|
A retrospective study by Gabriel, et al. showed that 24% of women with breast
implants had complications resulting in a reoperation during the first five years after implantation (silicone and saline
implants were studied together). 7
Prospective studies of saline-filled breast implants approved by FDA in May
2000 showed reoperation rates of 13-21% at 3 years and 20-26% at 5 years for augmentation patients. The same studies showed
reoperation rates of 39-40% at 3 years and 43-45% at 5 years for reconstruction patients.8,9
Photograph 1: Implant removal without replacement in augmentation patient
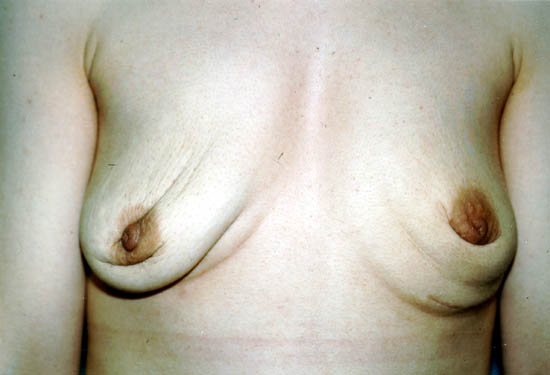
Photograph 2 below shows a 30-year-old woman’s left saline-filled
breast implant deflation.19 The suspected cause was the leaf-valve design of the implant, which is no longer being
used by manufacturers.
Photograph 2: Deflation
in augmentation patient
Silicone gel that escapes the scar capsule surrounding the implant may migrate away from the
breast. The free silicone gel may cause lumps called granulomas to form in the breast or in other tissues where the silicone
gel has migrated, such as the breast tissue, chest wall, armpit, or arm. Silicone gel may also migrate to distant organs such
as the liver. Migrated silicone gel may be difficult or impossible to remove.
Marotta, et al.22 reviewed numerous publications on over
9,770 silicone implants that were removed and concluded that 26% of implants were ruptured by 3.9 years, 47% were ruptured
by 10.3 years, and 69% were ruptured by 17.8 years. Marotta, et al. also reported that shells from removed silicone gel-filled
breast implants were considerably weaker than shells before implantation.
Capsular Contracture
Capsular contracture happens when the scar tissue or capsule that normally
forms around the implant tightens and squeezes the implant. It can happen to one or both of the implanted breasts.
There are four grades of capsular contracture - Baker grades I through
IV. The Baker grading is as follows:
|
Grade I |
the breast is normally soft
and looks natural |
|
Grade II |
the breast is a little firm
but looks normal |
|
Grade III |
the breast is firm and looks
abnormal |
|
Grade IV |
the breast is hard, painful,
and looks abnormal. |
The IOM report26 stated that, for studies involving both
silicone gel-filled and saline-filled breast implants, the capsular contracture rates were 36-81% for silicone-gel filled
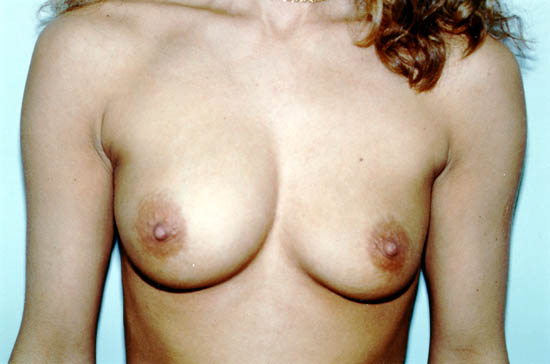
breast implants and 8-41% for saline-filled breast implants. Photograph 3 below shows grade IV capsular contracture in the right breast of a 29-year-old woman
seven years after subglandular placement of 560cc silicone gel-filled breast implants.29
Breast Pain
Prospective studies of saline-filled breast implants approved
by FDA in May 2000 showed breast pain rates of 5-16% at 3 years and 7-17% at 5 years for augmentation patients. The same studies
showed breast pain rates of 15-17% at 3 years and 16-18% at 5 years for reconstruction patients.31,32
loss of nipple sensation
in 12-35% of women at 3 years and in 18% of women at 5 years
6Safety
of Silicone Breast Implants. Institute of Medicine National Academy Press, Washington, D.C. 2000. {IOM Report}. Also available
through IOM website at www.iom.edu.
7Gabriel SE, Woods JE, O’Fallon WM, Beard CM, Kurland LT,
Melton LJ. Complications leading to surgery after breast implantation. New Engl J Med 1997; 336:679-682.
8Inamed
patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
9Mentor patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
10Inamed patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
11Mentor patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
12Brown SL, Pennello G. Replacement surgery and silicone gel breast
implant rupture: Self-report by women after mammoplasty. Journal of Women’s health & Gender Based Medicine, 2002;11:255-264.
A summary of the findings of this study is also available on FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
13Photograph courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C.,
University of Toronto.
14Although the term rupture is used for all types of implants, the term deflation is
typically used only for saline-filled breast implants.
15Betadine is a registered trademark of Purdue Frederick
Company.
16Safety of Silicone Breast Implants. Institute of Medicine National Academy Press, Washington, D.C.
2000. {IOM Report}. Also available through IOM website at www.iom.edu.
17 Inamed
patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
18Mentor patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
19Photograph courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C.,
University of Toronto.
20Safety of Silicone Breast Implants. Institute of Medicine National Academy Press, Washington,
D.C. 2000. {IOM Report}. Also available through IOM website at www.iom.edu.
21Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G. Prevalence
of rupture of silicone gel breast implants revealed on MR imaging in a population of women in Birmingham, Alabama. American
Journal of Roentgenology 2000;175:1-8. A summary of the findings of this study is also available on FDA’s website at
http://www.fda.gov/cdrh/breastimplants/.
22Marotta JS, Goldberg EP, Habal MB, Amery DP, Martin PJ, Urbaniak
DJ, Widenhouse CW. Silicone gel breast implant failures: Evaluation of properties of shells and gels for explanted prostheses
and meta-analysis of literature rupture data. Ann Plast Surg 2002;49:227-247.
23Holmich, LR, et al. Prevalence
of silicone breast implant rupture among Danish women. Plast Reconstr Surg. 2001; 108(4):848-858.
24Holmich,
LR, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003; 138:801-806.
25Safety of Silicone
Breast Implants. Institute of Medicine National Academy Press, Washington, D.C. 2000. {IOM Report}. Also available through
IOM website at www.iom.edu.
26Safety of Silicone Breast Implants. Institute of Medicine National
Academy Press, Washington, D.C. 2000. {IOM Report}. Also available through IOM website at www.iom.edu.
27Inamed patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
28Mentor patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
29Photograph courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C.,
University of Toronto.
30Safety of Silicone Breast Implants. Institute of Medicine National Academy Press, Washington,
D.C. 2000. {IOM Report}. Also available through IOM website at www.iom.edu.
31Inamed patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
32Mentor patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
33Inamed patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
34Mentor patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
35Inamed patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
36Mentor patient labeling at FDA’s website at http://www.fda.gov/cdrh/breastimplants/.
Updated June 8, 2004
Silicone Breast
Implants Available Under Tight Controls
By Marian Segal
at http://www.fda.gov/bbs/topics/CONSUMER/CON00146.html
Implants should not be expected to last a lifetime.
All implants "bleed" silicone gel through their outer envelope, and some percentage rupture, but that the health effects of the escape gel are uncertain. Since breast implants first came
on the market 30 years ago, an estimated 1-million women in the United States have had these devices surgically inserted. The percentage of implants that rupture is not certain. An FDA advisory panel concluded that the rupture rate may be higher than previously thought. Manufacturers’ report suggest a range between 0.2 and 1.1 percent; the medical literature contains
a range between 0 and 25%; and individual doctors said implants fail in as many as 32 percent of their patients.
The value of tests to detect silicone in blood and urine is uncertain. Since small amounts
of silicone "bleed" even from intact implants, these tests cannot tell whether your implant
has ruptured. Also, silicone is found in many products, including commonly used medicines
and cosmetics, so the source of silicone detected may not be clear. It is not known whether implants can cause or contribute
to the development of
connective tissue and immune-related disorders. But you should
be aware of symptoms that
can occur with these disorders. They include: -joint pain and swelling -skin tightness, redness or swelling -swollen glands
or lymph nodes-unusual and unexplained fatigue -swollen hands and feet -unusual hair loss
In recent years. However, there has been impassioned debate on the safety of silicone gel-filled
breast implants and whether or not the devices should remain on the market. Despite
the controversy, there was agreement among manufacturers, physicians, surgeons, consumer advocates, and women with implants
on at least one point: Solid clinical research is needed to answer questions
that loom large regarding the safety and long-term effects of these devices.
Saline filled implants are stil availabe without restriction
for both augmentation and reconstruction because leackage or rupture is not harmful.
|